Cholesterol at the Frontier of Science: New Insights and Implications
- Jacky Gao
- Oct 22, 2025
- 8 min read
Cholesterol has long been a key concept in cardiovascular health — especially the role of low-density lipoprotein cholesterol (LDL-C) often labelled the “bad cholesterol.” However, recent scientific advances are reshaping our understanding of cholesterol metabolism, atherosclerotic risk, and how lifestyle plus supplemental strategies may contribute to management.
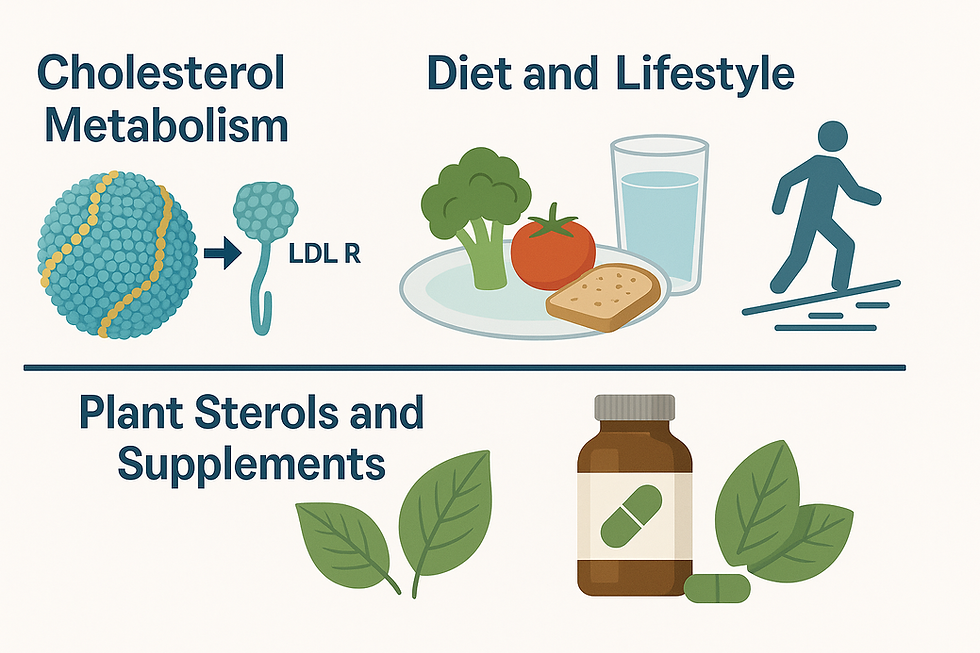
Evolving Mechanistic Understanding
Traditionally, elevated LDL-C levels were viewed simply as a risk marker for atherosclerotic cardiovascular disease (ASCVD). But recent mechanistic studies demonstrate that beyond the absolute LDL-C number, how LDL particles are handled by the body (receptor‐mediated clearance, penetration into the arterial wall, oxidative modification, small dense LDL vs large LDL) matters significantly.
For example, one line of research has elucidated how the LDL receptor (LDL R) binds to APOB100-containing LDL particles and mediates clearance. When this receptor–ligand interaction is impaired (by genetic variants or other dysfunction) LDL remains in circulation, infiltrates vessel walls and triggers plaque formation. This underscores that “cholesterol high = automatic high risk” is overly simplistic; the metabolic handling pathways are critical.
Further, particle heterogeneity has become an important focus. Smaller, denser LDL particles appear more atherogenic than larger buoyant ones. Some epidemiologic studies suggest that individuals with elevated LDL but favourable other markers (no inflammation, healthy metabolism, limited plaque burden) may not have as high a risk as once assumed. Thus, risk stratification is moving toward a multi‐parameter model (lipoprotein size/number, inflammation, endothelial function, plaque imaging) rather than LDL-C alone.
Changing Paradigms in Guidelines & Lifestyle Focus
Contemporary lifestyle guidance continues to emphasise diet, physical activity, weight control, smoking cessation, and avoidance of prolonged sedentary behaviour. What is shifting, however, is the emphasis: from merely reducing LDL-C numbers to improving metabolic health, reducing LDL particle burden, optimising lipoprotein profiles, and enhancing clearance/repair mechanisms.
Large observational datasets show that many patients fail to achieve guideline LDL-C targets despite therapy, and some may not even receive appropriate treatment. This gap underscores the need for improved translation of research to practice, including non‐drug adjunctive interventions.
Summary of Key Frontiers
The mechanisms of cholesterol clearance, LDL receptor function, and lipoprotein particle quality/size are increasingly seen as central to risk.
Risk heterogeneity: Two individuals with the same LDL-C may differ greatly in ASCVD risk due to differences in metabolism, inflammation, lipoprotein particle characteristics, and plaque burden.
Lifestyle remains foundational, but new adjuncts (dietary, botanical, supplement) are gaining attention as means to support cholesterol/lipoprotein management—not as replacements for proven therapies.
Botanical Extracts & Nutraceutical Ingredients in Cholesterol Management
With the above mechanistic nuance in mind, let us review several botanical extracts and nutraceutical ingredients that have been studied for their potential in cholesterol and lipoprotein management. Important caveat: these are adjunctive strategies — not replacements for physician-supervised lipid-lowering therapy when indicated.
Plant Sterols/Stanols (Phytosterols)
Mechanism: Plant sterols (phytosterols) are structurally similar to cholesterol. They compete with dietary and biliary cholesterol for incorporation into intestinal micelles and therefore reduce cholesterol absorption in the gut.
Clinical data: A meta-analysis of eight trials (4–6 weeks duration, doses ~1.0–3.0 g/day) found LDL-C was reduced by approximately 0.31 mmol/L (~12 mg/dL) compared with placebo. Another meta-analysis of 124 RCTs (mean ~2.1 g/day) found consistent dose-response — LDL-C reduction ~6-12% for intakes of 0.6–3.3 g/day.
Limitations and nuance: Although LDL lowering is consistent, there is no definitive evidence that phytosterol intake translates into reduced cardiovascular events (heart attacks, strokes). Moreover, the British Dietetic Association states: “While taking plant stanols and sterols has been shown to reduce cholesterol levels, there is insufficient long-term evidence to show they also reduce your risk of having a heart attack or stroke.”
Implications for product development / supplement use:
Typical effective dose ~2 g/day.
Format (fortified food vs supplement) may influence efficacy.
Should be adjunctive to diet/lifestyle.
Important to standardise extraction, ensure safety (e.g., sitosterolemia is a contraindication).
Communication must clarify: “reduces LDL by ~6–12%” and “no proven effect yet on clinical cardiovascular outcomes”.
Green Tea Extract / Tea Polyphenols
Mechanism: Green tea catechins (e.g., epigallocatechin gallate, EGCG) and theaflavin‐enriched tea extracts may influence lipid metabolism via multiple pathways: inhibition of cholesterol absorption, increased LDL receptor activity, antioxidant effects reducing LDL oxidation, and modulation of hepatic lipogenesis.
Clinical data:
A meta-analysis of 14 RCTs (1,136 subjects) found green tea reduced total cholesterol (TC) by ~7.2 mg/dL and LDL-C by ~2.19 mg/dL.
A later meta‐analysis of 34 trial arms found LDL reduction of approx. −5.80 mg/dL (95% CI −8.30 to −3.30) and slight HDL increase (WMD 1.85 mg/dL) in subgroup analyses.
A specific RCT: a 12-week capsule of theaflavin-enriched green tea extract (375 mg/day) in free-living adults on low saturated fat diet: TC down -11.3%, LDL-C down -16.4% vs placebo.
Limitations: The magnitudes of effect are modest (≈2-5% LDL reduction in many studies). Also, heterogeneity is significant (I² high in meta‐analyses), and effects may differ by baseline lipids, BMI, diet, gender, and diabetes status.
Implications:
Green tea extracts may serve as adjunctive support especially in individuals with mild to moderate dyslipidemia.
Clarify that expected LDL-C reduction is modest (<10%).
Emphasise synergy with dietary fat reduction, physical activity, weight control.
Safety and caffeine content should be considered (especially in individuals with arrhythmia, insomnia, medication interactions).
Additional Botanical/Herbal Candidates
While the evidence is less robust, other botanicals have also been studied:
Aged garlic extract (AGE): small trials suggest ~7% TC reduction, ~10% LDL-C reduction versus control in certain populations.
Bergamot (Citrus bergamia) polyphenols: pilot trials suggest beneficial changes in LDL-C and TC though sample sizes are small and studies heterogeneous.
Artichoke leaf extract, turmeric/curcumin, ginger, holy basil, etc.: some preliminary human or animal data suggest lipid-modifying effects, but evidence is far from definitive.
In all these cases, the same caveats apply: effect sizes moderate, study durations often short, and cardiovascular event data lacking.
Academic Analysis and Integration
Dose–Response and Population Variables
For phytosterols, meta‐analyses show a relatively clear dose–response relationship: intakes around 1.5–3 g/day produce LDL‐C reductions of ~8–12%. However, going beyond ~3 g/day appears to add little additional benefit. For tea extracts/catechins, the effect size appears smaller and more variable; baseline lipid levels, body mass index (BMI), gender, and comorbidities (e.g., type 2 diabetes) influence outcomes.
Mechanistic Considerations
Cholesterol homeostasis in humans involves: absorption from the gut, endogenous synthesis (via HMG-CoA reductase), receptor‐mediated clearance (LDL R), lipoprotein modification (oxidation, glycation), endothelial uptake, and reverse cholesterol transport (via HDL). Botanical extracts can intervene at some of these steps (e.g., phytosterols at absorption; tea catechins at absorption plus receptor activity plus anti‐oxidation).
But – unlike statins (which markedly reduce LDL-C via synthesis inhibition and have large outcome trials) – botanicals’ mechanisms tend to produce modest LDL‐C changes, and the ‘downstream’ impact on plaque burden, cardiovascular event reduction, and long–term safety is less well characterised.
Evidence vs Outcome Gaps
A key academic caveat: reduction in LDL-C is considered a surrogate marker for cardiovascular risk, but the definitive standard is reduction in hard outcomes (myocardial infarction, stroke, cardiovascular mortality). For many of these botanicals, such outcome trials are absent or very limited. For example, with phytosterols: “there are no data indicating that the consumption of phytosterols may reduce the risk of CVD.” This means while LDL‐C reductions suggest a favourable effect, we cannot claim proven risk reduction.
Integration into Practice and Product Development
From a product development or corporate website perspective, important points include:
Transparent disclosure of effect size (e.g., “in trials ~6-12% LDL reduction for ~2 g/day phytosterols”) rather than overselling.
Emphasise that these are adjuncts to lifestyle (diet/exercise) and not replacements for medically indicated lipid-lowering therapy.
Highlight that individuals with very high LDL-C, familial hypercholesterolemia, or established cardiovascular disease should follow physician-directed therapy.
Consider format, dosage, standardisation and quality control of botanical extracts.
Include safety/contraindications (see next section).
Focus on synergy: specifying that botanical adjuncts perform best when combined with dietary saturated fat reduction, increased physical activity, weight control and smoking cessation.
Emphasise that botanical interventions are best suited for mild to moderate dyslipidemia or as part of preventive strategies, rather than as monotherapy in high‐risk patients.
References (selected)
AbuMweis SS, et al. “Cholesterol-lowering efficacy of plant sterols/stanols provided in the context of a dietary portfolio of cholesterol-lowering foods: a meta-analysis.” Eur J Clin Nutr. 2013;67(3):264-271. Meta-analysis showed LDL-C decrease by ~12 mg/dL.
Demonty I, et al. “Plant Sterols and Plant Stanols in Cholesterol Management and Atherosclerosis Research.” Curr Atheroscler Rep. 2015;17(3):496. Reports meta-analyses of ~124 RCTs showing LDL-C reductions of 6–12% with 0.6–3.3 g/day.
Li A, Wang Q, Li P et al. “Effects of green tea on lipid profile in overweight and obese women: a systematic review and meta-analysis of RCTs.” Int J Vit Nutr Res. 94(3/4): (2019) … Weighted mean differences showed ~2-5% reductions.
Nagao T, Hase T, Tokimitsu I. “Cholesterol-Lowering Effect of a Theaflavin-Enriched Green Tea Extract.” JAMA Intern Med. 2003;163(9):1448-53. Shows −11.3% TC and −16.4% LDL-C in 12-week RCT.
National Center for Complementary and Integrative Health (NCCIH). “High Cholesterol and Natural Products: What the Science Says.” (2019) Reviews data and notes limitations of outcome evidence.
WebMD. “Plant sterols.” Reviews mechanism, potential LDL-C lowering but also notes limited long‐term data.
Cleveland Clinic. “Phytosterols (plant sterols/stanols)”. Overview for public on this ingredient.
Risks, Safety Considerations & Disclaimers
Not a substitute for medical treatment: Individuals with very high LDL-C, familial hypercholesterolemia, existing cardiovascular disease, or those on lipid-lowering medications must follow their physician’s guidance. Botanical extracts and nutraceuticals should be adjuncts, not replacements.
Effect size modest: Typical LDL-C reductions with phytosterols ~6–12%; with tea extracts ~2–5%. Do not oversell.
Outcome data lacking: While LDL-C reduction is a favourable surrogate, there is currently insufficient evidence that many of these supplements reduce actual cardiovascular events (heart attack, stroke).
Potential interactions and contraindications:
Phytosterols may interfere with absorption of fat-soluble nutrients or bile acid sequestrants. Persons with rare genetic condition sitosterolemia (phytosterol accumulation) should avoid plant sterol supplements.
Green tea extract contains caffeine and may impact sleep, blood pressure, heart rhythm, or interact with medications (e.g., anticoagulants).
Quality/consistency: Botanical supplement markets are variable; standardisation, extraction method, contaminant testing matter.
Lifestyle first: These supplements do not replace foundational lifestyle interventions: diet (saturated fat reduction, increased fibre, plant-rich), physical activity, weight control, smoking cessation.
Individual variability: Response to supplements varies widely depending on baseline lipids, genetics, BMI, comorbidity (e.g., diabetes), diet, and lifestyle.
Regulatory status: In many jurisdictions, supplements are not held to the same rigorous standard as pharmaceuticals (no requirement for large outcome trials). Claims must be worded carefully.
Pregnancy, lactation, children: Safety data are limited in these populations. Generally advisable to avoid or use under specialist supervision.
Expectations management: Communicate that these are adjunct supports, and “more is not always better” (e.g., >3 g/day of phytosterols has limited added effect).
Conclusion
In summary:
Our scientific understanding of cholesterol and atherosclerotic risk is evolving from simple “LDL high = high risk” toward a nuanced model that includes lipoprotein particle characteristics, metabolism, inflammation and plaque burden.
Botanical and nutraceutical interventions (e.g., plant sterols/stanols, green tea extracts) offer adjunctive support for lipoprotein management, with modest but measurable LDL-C reductions in controlled settings.
These interventions are not replacements for physician-directed lipid-lowering therapy when indicated, nor for robust lifestyle changes.
From a corporate product or communications perspective, it is critical to present transparent data, clarify scope and limitations, ensure quality & safety, and position the ingredients as part of a whole-system approach (diet + exercise + lifestyle + adjunctive support).
Future frontier: greater individualisation of risk profiling (genetics, lipoprotein subclass analysis, imaging), and integration of botanical/nutraceutical interventions into stratified preventive care.












